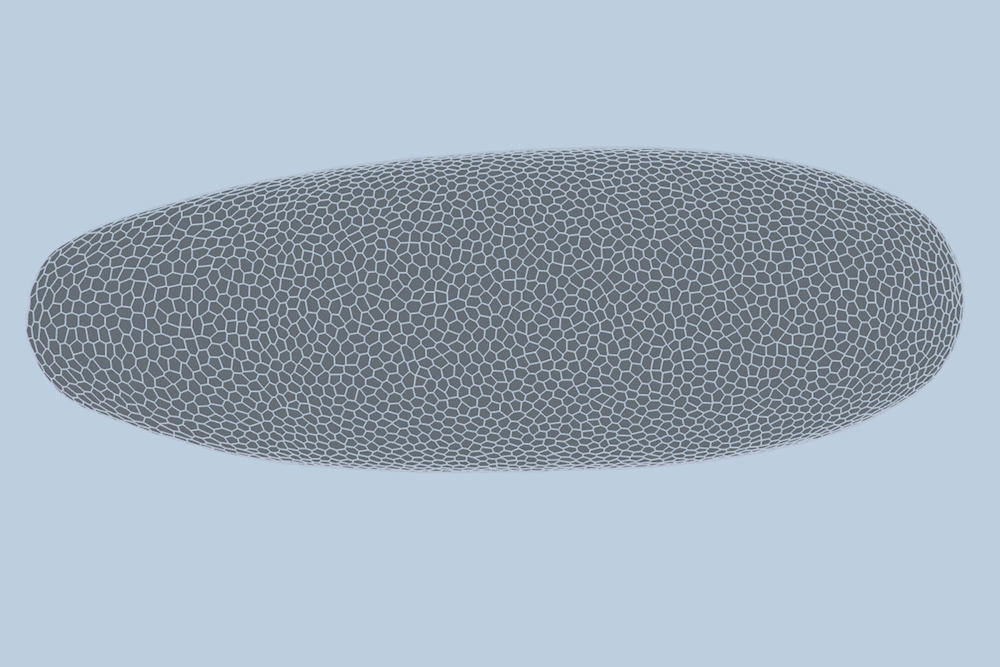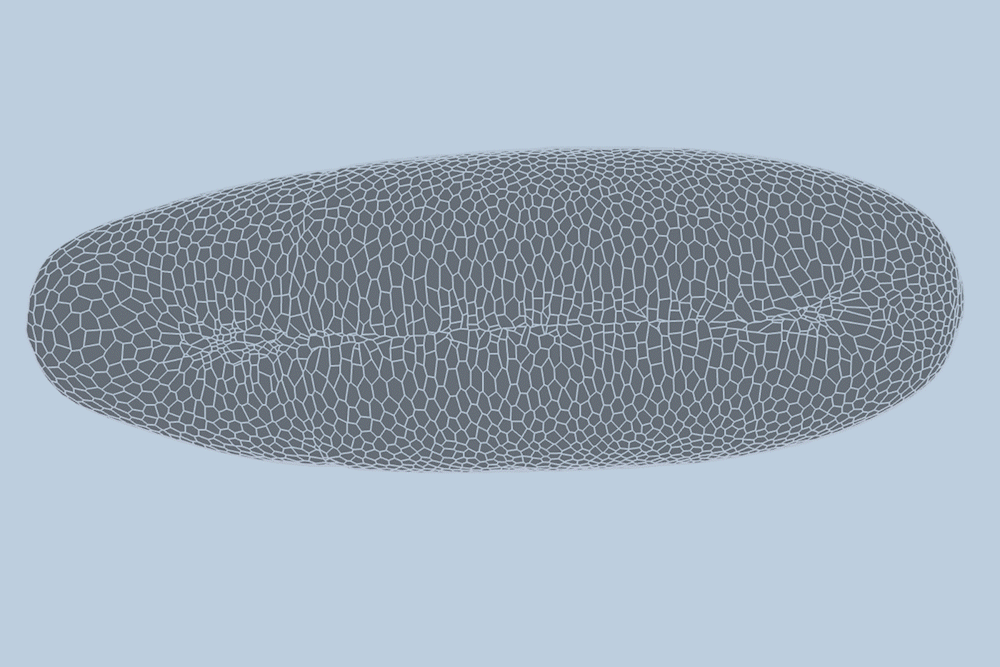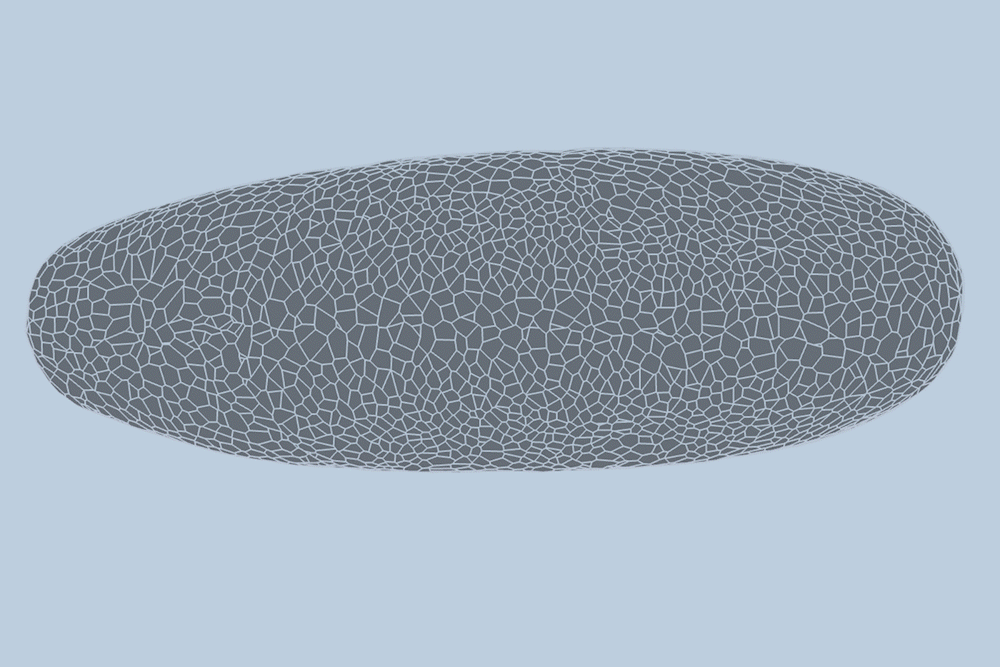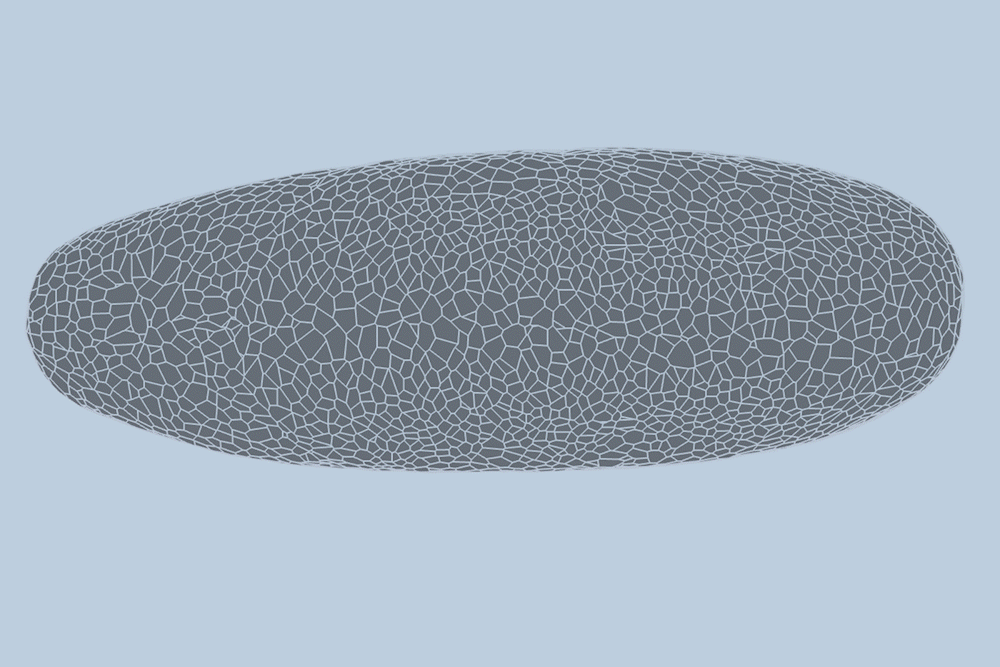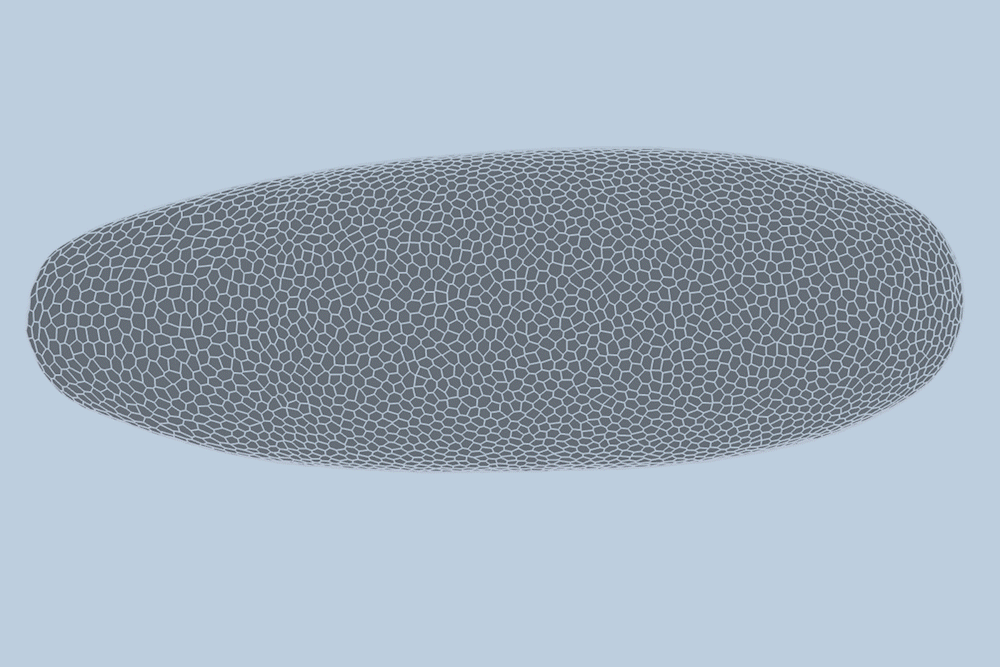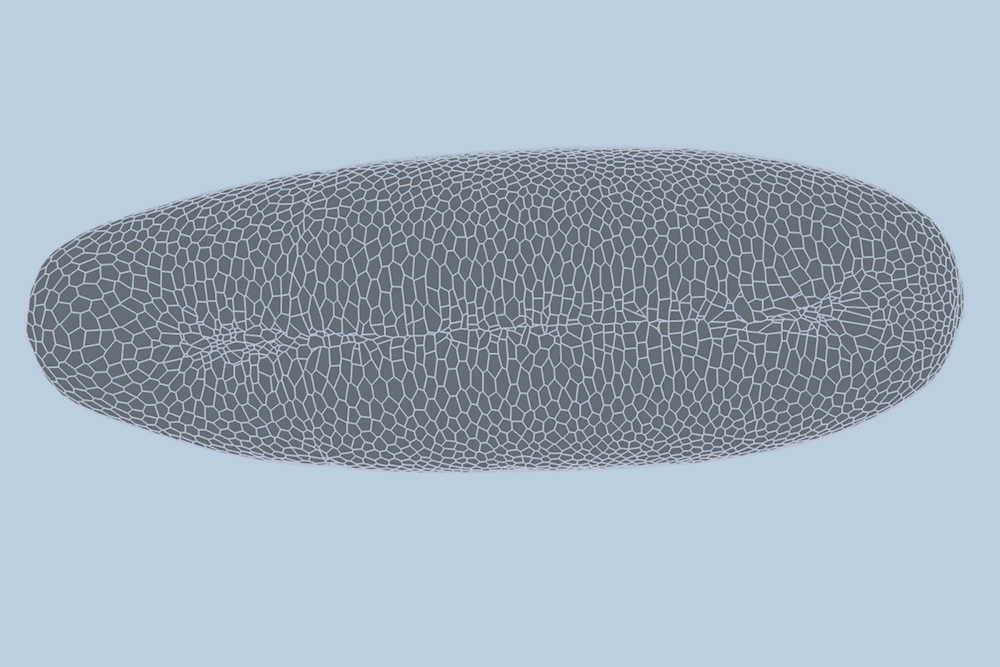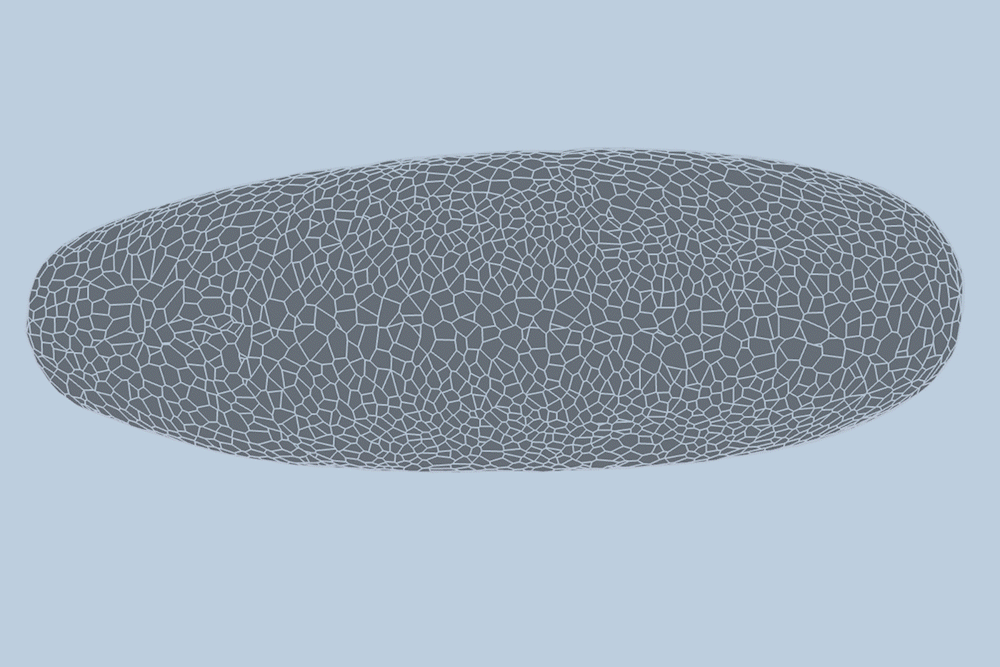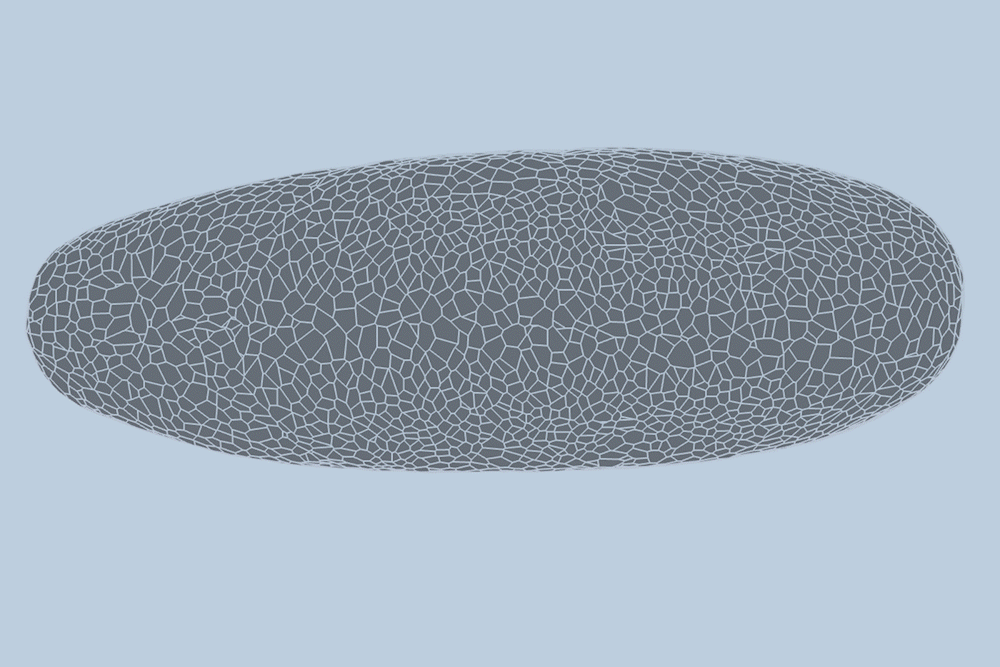
During the early stages of development, tissues and organs begin to bloom as thousands of cells move, divide, and grow.
A team of MIT engineers has now developed a minute-by-minute method to predict how individual cells will fold, divide, and rearrange themselves during the early stages of fruit fly development. This new method may one day be applied to predict the development of more complex tissues, organs, and organisms. It could also help scientists identify cell patterns that correspond to early-onset diseases such as asthma and cancer.
In a study published today in the journal nature methodthe team presents a new deep learning model that learns and predicts how specific geometric properties of individual cells change as fruit flies grow. The model records and tracks properties such as a cell’s position and whether it is touching neighboring cells at a given moment.
The research team applied this model to videos of developing Drosophila embryos. Each embryo begins as a collection of about 5,000 cells. They found that the model could predict with 90% accuracy minute by minute how each of its 5,000 cells will fold, move, and rearrange itself during the first hour of development as the embryo transforms from a smooth, uniform shape to more defined structures and features.
“This very early step, known as gastrulation, occurs over about an hour, during which individual cells rearrange themselves on a time scale of minutes,” says study author Ming Guo, an MIT associate professor of mechanical engineering. “By accurately modeling this early period, we can begin to reveal how local cellular interactions give rise to tissues and organisms across the planet.”
The researchers hope to apply this model to predict cell-by-cell development in other species, such as zebrafish and mice. We can then begin to identify common patterns across species. The research team also envisions that the method could be used to identify early patterns in diseases such as asthma. The lung tissue of asthma patients looks markedly different from healthy lung tissue. How asthma-prone tissue first develops is an unknown process that the researchers’ new method could shed light on.
“Asthmatic tissues exhibit different cellular dynamics when imaged live,” says co-author and MIT graduate student Haiqian Yang. “We believe this model captures these subtle mechanical differences and provides a more comprehensive representation of tissue behavior, with the potential to improve diagnostic and drug screening assays.”
Co-author of the study is Markus Bühler, McAfee Professor of Engineering in the Massachusetts Institute of Technology’s Department of Civil and Environmental Engineering. George Roy and Tomer Stern of the University of Michigan; Anh Nguyen and Dapen Bi of Northeastern University;
points and forms
Scientists typically model how embryos develop in one of two ways. One is as a point cloud, where each point represents an individual cell as a point moving over time. Or as “bubbles,” which represent individual cells as bubbles that shift or slide around each other, similar to the foam in shaving foam.
Rather than choosing between the two approaches, Guo and Yang adopted both approaches.
“There is a debate about whether to model it as a point cloud or as a bubble,” Yang says. “But these are essentially different ways of modeling the same underlying graph, which is a sophisticated way of representing living tissue. By combining them as a single graph, we can highlight more structural information, such as how cells connect to each other as they rearrange themselves over time.”
At the heart of the new model is a “dual graph” structure that represents the developing embryo as both a moving point and a bubble. Through this dual representation, the researchers hoped to capture more detailed geometric properties of individual cells, such as the location of the cell’s nucleus, whether the cell is in contact with neighboring cells, and whether it is folding or dividing at a given moment.
As a proof of principle, the team trained a new model to “learn” how individual cells change over time during Drosophila gastrulation.
“The overall shape of the Drosophila at this stage is almost ellipsoid, but huge dynamics are occurring at the surface during gastrulation,” Guo says. “It goes from being completely smooth to forming many folds at different angles. And we want to predict all those dynamics, moment by moment, cell by cell.”
when and where
For the new study, the researchers applied their new model to high-quality videos of Drosophila gastrulation taken by collaborators at the University of Michigan. The video is a 1-hour recording of a developing Drosophila melanogaster taken at single-cell resolution. Additionally, the videos include labeling of the edges and nuclei of individual cells, which is incredibly detailed and difficult to obtain data.
“These videos are very high quality,” Yang says. “This data is extremely rare and provides submicron resolution of the entire 3D volume at fairly fast frame rates.”
The researchers trained a new model using data from three out of four videos of fruit fly embryos, allowing the model to “learn” how individual cells interact and change as the embryo grows. They then tested the model on a brand new Drosophila video and found that it could predict with high accuracy how most of the embryo’s 5,000 cells change from minute to minute.
Specifically, the model can predict the properties of individual cells, such as whether they will fold, split, or continue to share edges with neighboring cells, with about 90% accuracy.
“We end up predicting not just whether these things will happen, but when they will happen,” Guo said. “For example, will this cell detach from this cell seven minutes from now, or eight minutes from now? We can tell when that’s going to happen.”
The researchers believe that, in principle, the new model and dual-graph approach should be able to predict cell-by-cell development in other multicellular systems, such as more complex species and some human tissues and organs. The limiting factor is the availability of high quality video data.
“From a model perspective, I think we’re ready,” Guo said. “The real bottleneck is data. If we have high-quality data for a particular tissue, we can directly apply that model to predict the development of many more structures.”
This research was supported in part by the National Institutes of Health.