To generate effective targeted therapies for cancer, scientists need to isolate the genetic and phenotypic properties of cancer cells both within and within different tumors.
This study requires a deeper understanding of the RNA or protein molecules that each cancer cell expresses, the location of the tumor, and its appearance under a microscope.
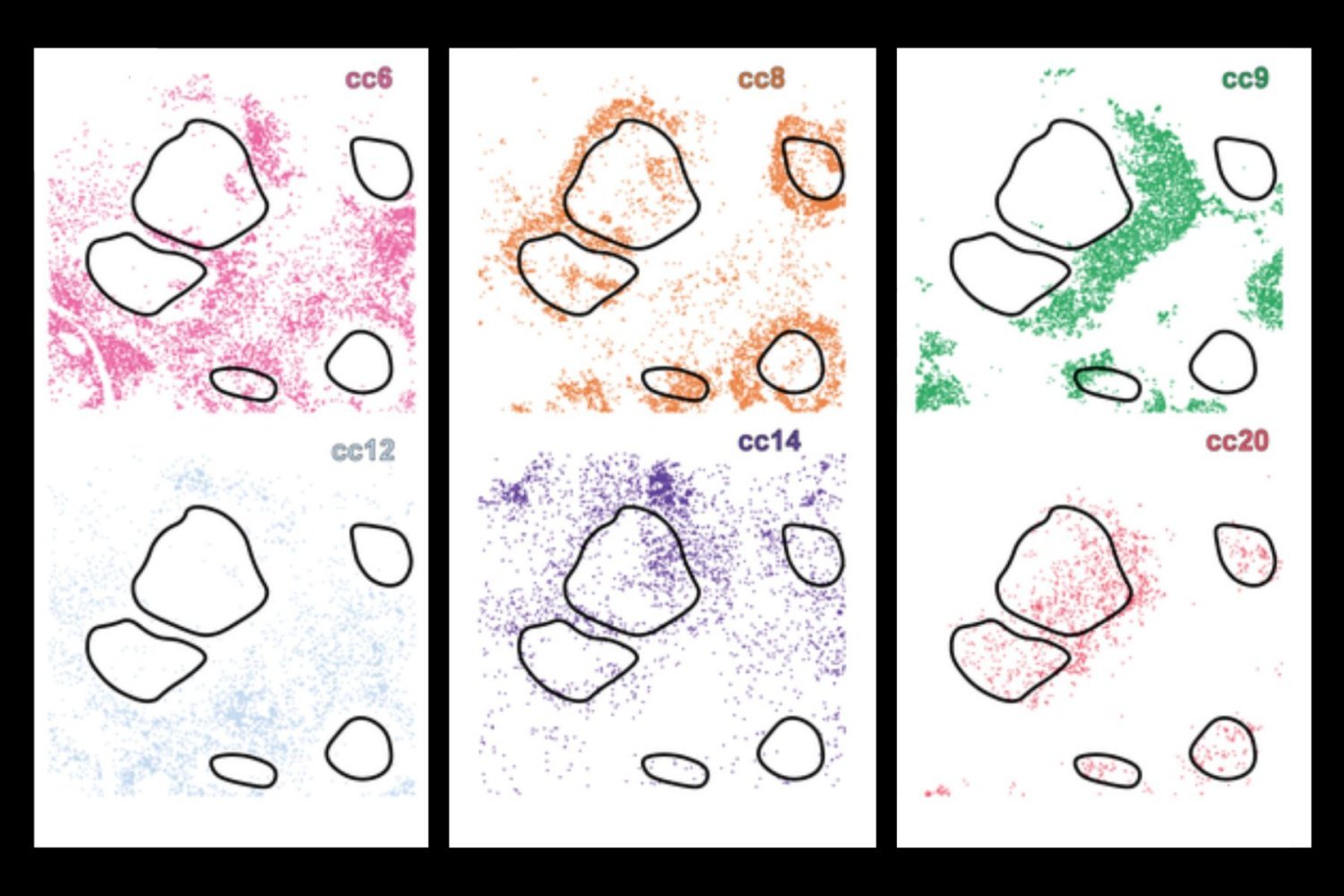
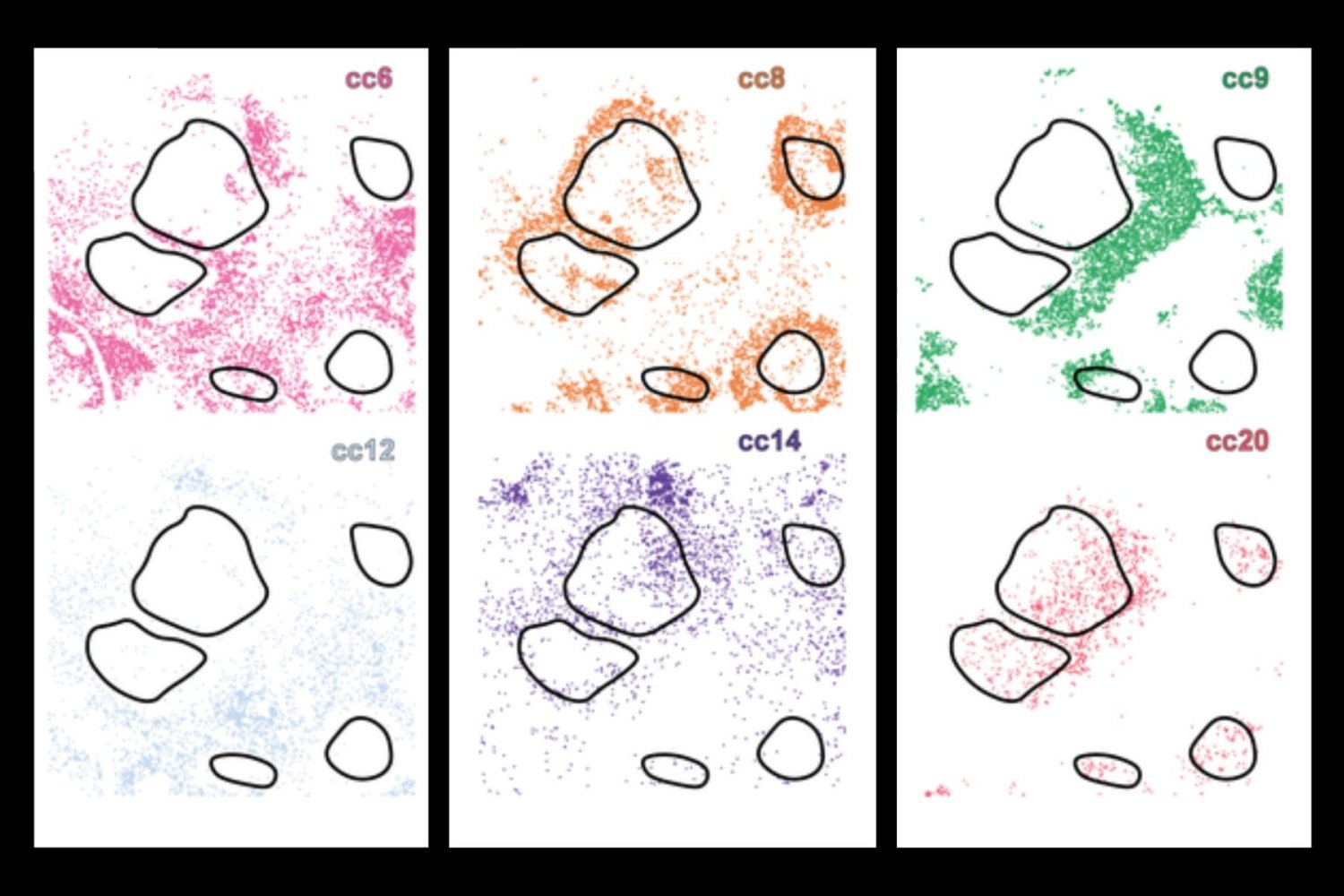
Traditionally, scientists have looked into one or more of these aspects individually, but now the new deep learning AI tool, Celllens (Cell Local Environment and Neighborhood Scan), uses a combination of convolutional and graph neural networks to build a comprehensive digital profile for every cell. This allows the system to group cells with similar biology. This effectively separates different behaviors, although they appear to be very similar in isolation.
Recently published research Innate Immunologydetailing the results of collaborations with researchers from MIT, Harvard Medical School, Yale University, Stanford University and the University of Pennsylvania. This is an effort led by MIT Postdock and MGH, Harvard University, MGH, MIT, and Bokai Zhu, a member of Harvard’s Lagon Institute.
Zhu explains the impact of this new tool. “Initially, we found a cell. This is called a T cell. This is using the same data set and applying Celllens, which is a T cell, and is currently attacking a specific tumor boundary in a patient.
“We can use existing information to better define what a cell is, a subpopulation of that cell, what it is, what it is doing, and what its potential functional reads are. This method can be used to provide specific detailed information about a cell in the disease and to identify new biomarkers that allow for the development of more targeted, measured therapies.”
This is an important advance, as current methodologies often overlook important molecular or contextual information. For example, immunotherapy may target cells that are only present at the borders of a tumor, limiting their efficacy. Using deep learning, researchers can use cellenes to detect different layers of information, such as morphology and locations in tissues spatially.
When applied to samples from healthy tissues and several types of cancer, such as lymphoma and liver cancer, Serulen discovered rare immune cell subtypes and revealed how their activity and location are related to disease processes such as tumor invasion and immunosuppression.
These findings could help scientists to better understand how the immune system interacts with tumors and pave the way for more accurate cancer diagnosis and immunotherapy.
“We are extremely excited by the potential of new AI tools like Celllens, which will help us to understand a more comprehensive understanding of abnormal cellular behavior within our tissues,” says co-author Alex K. Shalek. Members of the Broad and Lagon Institutes. “Now we can measure a huge amount of information about the context of individual cells and their tissues in state-of-the-art multi-omicus assays. Effective use of that data to nominate new therapeutic leads is when we promise to promote human health and ability to withstand human health and wellbeing, when combined with appropriate input data and the activation of careful downstopping.”